

MICROFLUIDICS
Flow-chamber-based thrombus formation:
from research tool to diagnostic test.
“Stop stirring, start flowing!”
Blood platelets are essential to human life. One of the primary functions of platelets is to maintain vascular integrity and to stop bleeding. The formation of a stable platelet plug depends on the ability of the platelet to interact with the damaged vascular bed, recruitment of other cells and function as a phospholipid surface for the activation of the coagulation cascade in order for fibrin to be formed which stabilizes the plug.
Defects in this process can cause bleeding symptoms, ranging from clinically insignificant to severe. Platelet defects can be classified as defects in adhesion, activation and aggregation or more based on their particular structural or functional deficiency. Inherited defects of platelet function are heterogenous and can result in bleeding symptoms ranging from mild bruising to severe mucocutaneous hemorrhage.
Several methods are available to measure platelet function and they all have different detection mechanisms, use different samples preparation and have specific advantages and limitations. Most platelet function tests focus on one or two aspects of platelet function and are not performed in a physiological environment such as whole blood or venous/arterial flow. Light transmission aggregometry (LTA) in platelet rich plasma is still the gold standard and is advised in several guidelines (CLSI, ISTH-SSC,[1]) in the workup of thrombopathy in combination with a nucleotide assay to detect storage pool disease. Other methods such a whole blood multiple electrode aggregation assays (MEIA) [2] or flowcytometry [3] are suggested as alternatives. Accordingly, shear-dependent defects of platelets are likely not all detected in these static (or stirring) tests of hemostasis.
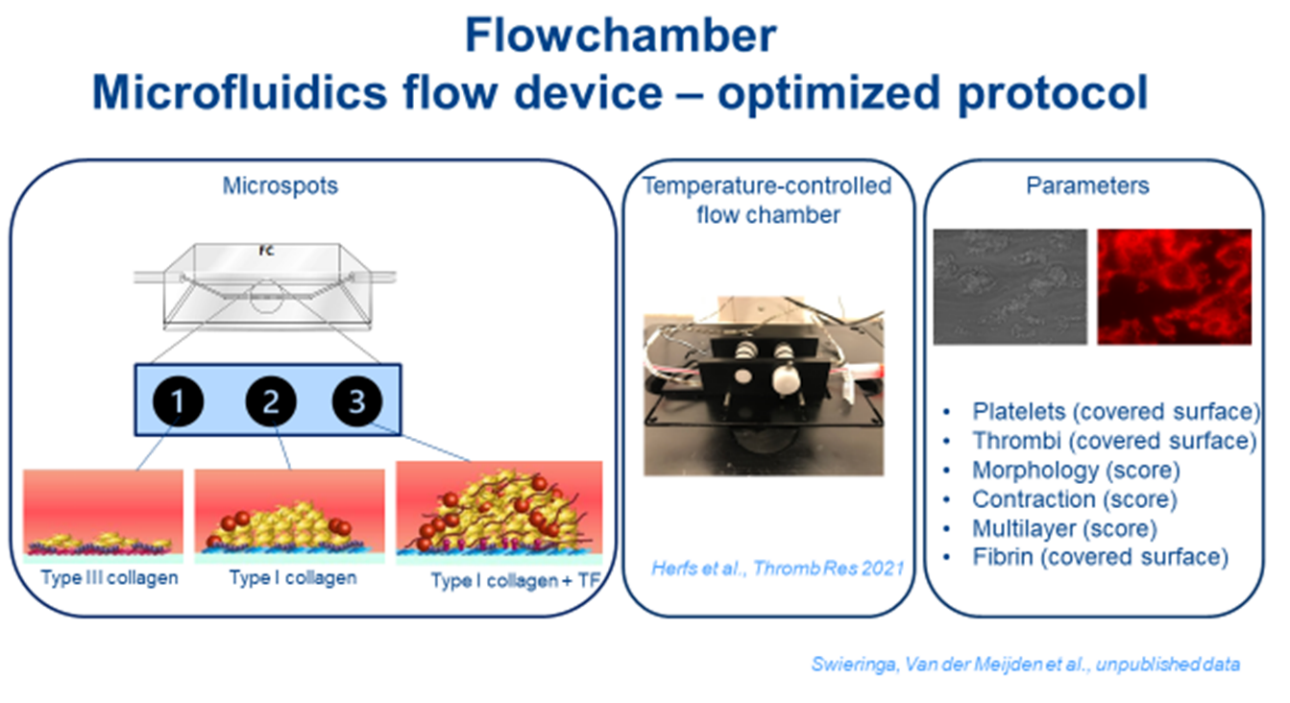
Given the multiple interactions between platelets, coagulation and vascular components during thrombus formation, the simultaneous assessment of all these processes under flow would be the ideal way to detect hemostatic abnormalities. The first flow dependent, automated platelet function analyzer (PFA) has been developed as a proxy measurement of primary hemostasis under conditions of extreme high wall-shear rate. However, use of the PFA test in identifying patients with mild bleeding disorders has been questioned in studies [2] and meta-analyses and the PFA result offers only a closure time and no information on the cause of the defect (VWF or platelets).
Recently, other flow dependent point of care devices have become available: the Global Thrombosis Test (GTT) and the Total-Thrombus-formation Analysis System (T-TAS) to monitor thrombus formation. More advanced, microfluidic whole-blood flow assays are described and used to study platelet thrombus formation in research settings. These multiparameter measurements of thrombus formation and fibrin formation give a lot of information about platelet adhesion, activation and aggregation and fibrin formation under flow conditions, especially when combined with arrays of microspots (containing different types of collagens with or without tissue factor) in the same flow chamber. Furthermore, these systems are very suitable for developing “the vessel on chip” principle by coatings with (damaged) endothelial cells [4]. In combination with the use of brightfield and multicolor fluorescence microscopy, the microfluidic assay thus produces multiple platelet-dependent outcome values relevant for flow conditions. We are leading in complex platelet phenotyping and unravelling acquired or inherited bleeding conditions in patients using these flow chambers [5,6] and recently also with an in-house developed 37 degrees Celsius flow chamber [7]. Moreover, we recently showed that patients with evident bleeding symptoms, and an ‘unexplained’ prolonged PFA-CT as the only abnormality found, appear to have different flow related abnormalities after retesting in a microfluidic device [8].
To get these promising flow chamber methods [9] ready for daily clinical purposes more research and new developments are needed on the method of clot detection, automated data analysis, standardization of (pre)analytics and extensive clinical validation. Our overall aim is to map the hemostatic balance of bleeding patients in an acute clinical setting by integrating innovative diagnostic tools and clinical parameters. For this we have set up the Hemosum platform in which we foster research, education and business initiatives on global hemostasis tests for improving bleeding risk prediction and treatment for patients with a hereditary or acquired hemostatic disorder.
References
1. Munnix ICA, Van Oerle R, Verhezen P, Kuijper P, Hackeng CM, Hopman-Kerkhoff HIJ, Hudig F, Van De Kerkhof D, Leyte A, De Maat MPM, Oude Elferink RFM, Ruinemans-Koerts J, Schoorl M, Slomp J, Soons H, Stroobants A, Van Wijk E, Henskens YMC. Harmonizing light transmission aggregometry in the Netherlands by implementation of the SSC-ISTH guideline. Platelets. 2021 May 19;32(4):516-523.
2. Moenen FCJI, Vries MJA, Nelemans PJ, van Rooy KJM, Vranken JRRA, Verhezen PWM, Wetzels RJH, Ten Cate H, Schouten HC, Beckers EAM, Henskens YMC. Screening for platelet function disorders with Multiplate and platelet function analyzer. Platelets. 2019;30(1):81-87.
3. van Asten I, Schutgens REG, Baaij M, Zandstra J, Roest M, Pasterkamp G, Huisman A, Korporaal SJA, Urbanus RT. Validation of flow cytometric analysis of platelet function in patients with a suspected platelet function defect. JTH. 2018 Apr;16(4):689-698.
4. Brouns SLN, Provenzale I, van Geffen JP, van der Meijden PEJ, Heemskerk JWM. Localized endothelial-based control of platelet aggregation and coagulation under flow: A proof-of- principle vessel-on-a-chip study. J Thromb Haemost. 2020 Apr;18(4):931-941.
5. de Witt SM, Swieringa F, Cavill R, Lamers MM, van Kruchten R, Mastenbroek T, Baaten C, Coort S, Pugh N, Schulz A, Scharrer I, Jurk K, Zieger B, Clemetson KJ, Farndale RW, Heemskerk JW, Cosemans JM. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014 Jul 16;5:4257.
6. Swieringa F, Baaten CC, Verdoold R, Mastenbroek TG, Rijnveld N, van der Laan KO, Breel EJ, Collins PW, Lancé MD, Henskens YM, Cosemans JM, Heemskerk JW, van der Meijden PE. Platelet Control of Fibrin Distribution and Microelasticity in Thrombus Formation Under Flow. Arterioscler Thromb Vasc Biol. 2016 Apr;36(4):692-9.
7. Herfs L, Swieringa F, Jooss N, Kozlowski M, Heubel-Moenen FCJ, van Oerle R, Machiels P, Henskens Y, Heemskerk JWM. Multiparameter microfluidics assay of thrombus formation reveals increased sensitivity to contraction and antiplatelet agents at physiological temperature.Thromb Res. 2021 Jul;203:46-56.
8. Heubel-Moenen FCJI, Brouns SLN, Herfs L, Boerenkamp LS, Jooss NJ, Wetzels RJH, Verhezen PWM, Machiels P, Megy K, Downes K, Heemskerk JWM, Beckers EAM, Henskens YMC. Multiparameter platelet function analysis of bleeding patients with a prolonged platelet function analyser closure time Br J Haematol. 2022 Mar;196(6):1388-1400.
9. Provenzale I, Brouns SLN, van der Meijden PEJ, Swieringa F, Heemskerk JWM. Whole Blood Based Multiparameter Assessment of Thrombus Formation in Standard Microfluidic Devices to Proxy In Vivo Haemostasis and Thrombosis. Micromachines (Basel). 2019 Nov 16;10(11):787. doi: 10.3390/mi10110787. PMID: 31744132; PMCID: PMC6915499.
